The #1 Percutaneous MIS Bunion Company in the US*
Instrumented Reproducibility
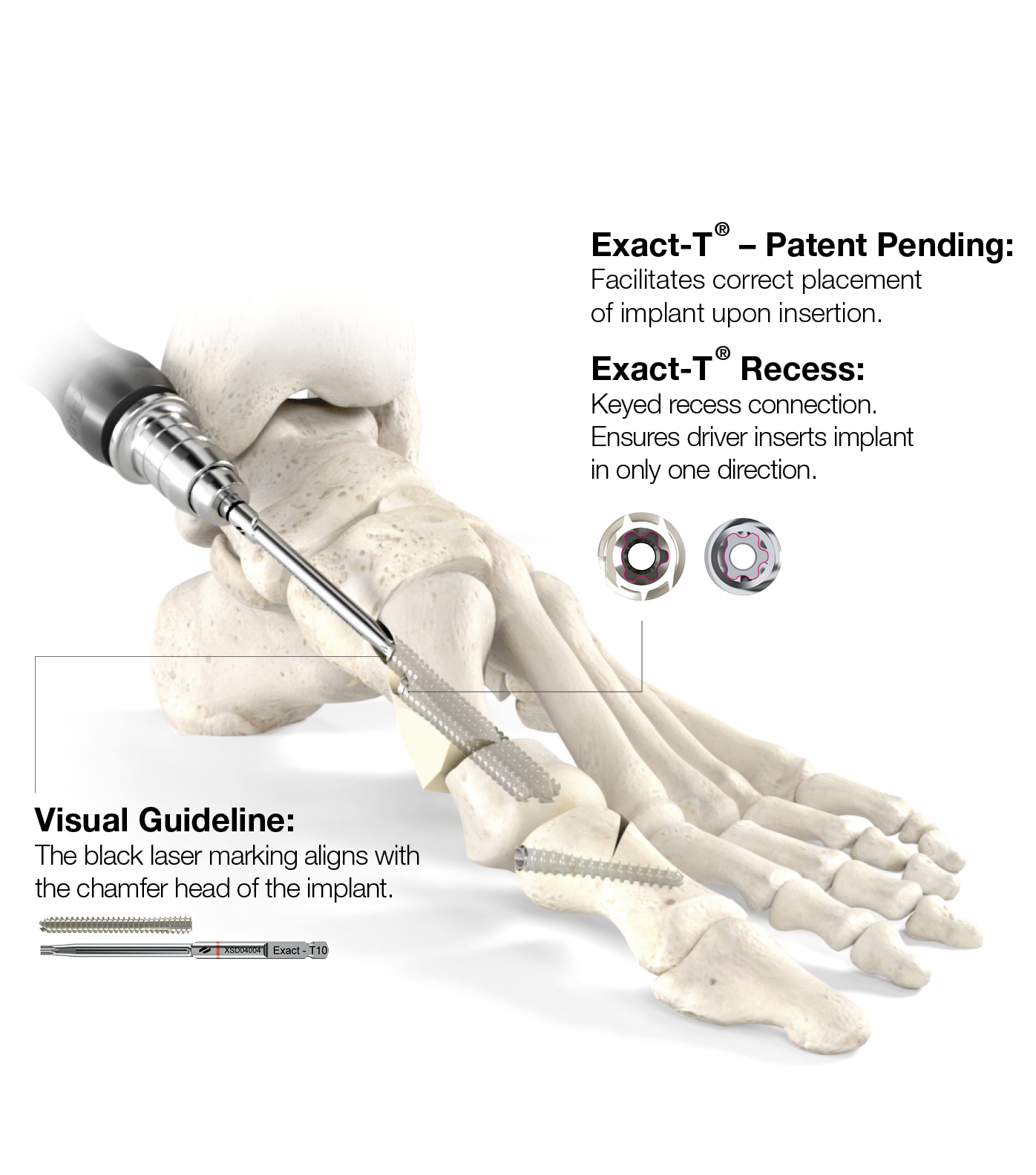
Technology
Ready to upgrade your Hallux Valgus corrections?
Novastep recognizes the apparent steady plateau of products in the foot and ankle industry and offers an innovative solution. The Pecaplasty® System¹ is a dedicated platform that allows for a minimally invasive correction of hallux valgus and other deformities in the foot and ankle.

Technology
Power of a lapidus without the fusion
The debate over the effectiveness of minimally invasive vs traditional open incisions techniques when correcting hallux valgus, lead Novastep to formulate a powerful concept. Centrolock¹
a guided transverse osteotomy system, combines the benefits of minimally invasive surgery with powerful triplanar correction.
a guided transverse osteotomy system, combines the benefits of minimally invasive surgery with powerful triplanar correction.

Technology
When Static Compression Isnt Enough
As the Lapidus technique continues to be a mainstay in correcting hallux valgus, the challenges surrounding the procedure remain constant. The Lapi-Arc system was designed to facilitate tri-planar correction. The Targeting Arc to achieve accurate placement of the lag screw for maximum compression across the tarsometatarsal joint.
* Trailing 12mo. revenue based on company market estimates.
¹ Patent pending.
TECHNOLOGY DRIVEN FOCUS
Leading in innovation of disruptive technology
- Enhanced Manufacturing processes
- Reduced Healthcare Costs
- Enriched Delivery Experience
- Resolved Clinical Hurdles
Ambulatory Surgery Center (ASC) Solutions
Cost-effective, positive outcomes
Novastep focuses on health and economic data to help provide up-to-date product information to Value Analysis Committees, clinicians, GPO’s, integrated delivery networks, and other stakeholders at Ambulatory Surgery Center (ASC) facilities.
This data includes both clinical and economic evidence to support Novastep products and typically demonstrates how the company’s product line may add value by helping to solve clinical hurdles, improve product delivery experience and reduce healthcare costs.


Manage or
Reduce
COSTS
Reduce
COSTS
Improve the
Product
DELIVERY
EXPERIENCE
Product
DELIVERY
EXPERIENCE
Achieve Better
Healthcare
Through
Improved
OUTCOMES
Healthcare
Through
Improved
OUTCOMES




























 US site
US site
 INT site
INT site
 FR site
FR site